Type what you are searching for:
- Certification of compliance CE approved and registered at the Italian Ministry of Health
- Packaging for a single machine
- AFS 1000 chromatographic immunofluorescence machine
- Multi-diagnostic 4D scanner that can be used for other tests
- Automatic printing of the test report
- Touchscreen for faster use
- Activates in 10 seconds
Download data sheet
- Certification of compliance CE approved and registered at the Italian Ministry of Health
- Packaging for a single machine
- AFS 1000 chromatographic immunofluorescence machine
- Multi-diagnostic 4D scanner that can be used for other tests
- Automatic printing of the test report
- Touchscreen for faster use
- Activates in 10 seconds
Download data sheet
- Certification of compliance CE approved and registered at the Italian Ministry of Health
- Packaging for a single machine
- AFS 1000 chromatographic immunofluorescence machine
- Multi-diagnostic 4D scanner that can be used for other tests
- Automatic printing of the test report
- Touchscreen for faster use
- Activates in 10 seconds
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by ELDO CARE CORPORATION LIMITED
- Box of 50 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 25 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 50 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 25 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 50 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 50 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Dispositivo Medico conforme a 93/42/ECC MDD Annex VII
- Conforme EN 14683:2019+AC:2019
- Certificato di Conformità CE rilasciato da OBELIS
- Scatola da 50 pezzi in una unica confezione
- Mascherina a tre strati con strato interno filtrante in tessuto “non tessuto”
- Resistenza ai fluidi 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 50 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 50 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 50 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 50 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 50 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 50 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Medical device compliant with 93/42/ECC MDD Annex VII
- Compliant with EN 14683:2019+AC:2019
- CE certificate issued by OBELIS
- Box of 50 pieces
- Each mask is Individually packaged
- Mask has three layers with an internal filtering layer in “non woven” fabric
- Resistance to fluids 16kPa (120 mmHg)
Download data sheet
- Each mask is Individually packaged
- KN95/ FFP2 protection class III category
- CE certificate issued UE 425/2016 e D.Lgs 17/2019
- Production standards in compliance with the regulation EN149:2001+A1:2009
- 5-ply mask with double internal filtering layer in “meltblown”
- Filtration efficiency ≥95%
- Box of 20 pieces in single packs of 5 pieces each.
Download data sheet
- Each mask is Individually packaged
- KN95/ FFP2 protection class III category
- CE certificate issued UE 425/2016 e D.Lgs 17/2019
- Production standards in compliance with the regulation EN149:2001+A1:2009
- 5-ply mask with double internal filtering layer in “meltblown”
- Filtration efficiency ≥95%
- Box of 20 pieces in single packs of 5 pieces each.
Download data sheet
- Each mask is Individually packaged
- KN95/ FFP2 protection class III category
- Production standards in compliance with the regulation EN149:2001+A1:2009
- 4-ply mask with internal filtering layer in “non-woven” fabric
- Filtration efficiency ≥95
- box of 20 pieces in single packs of 50 pieces each.
- Product marketed in derogation from INAIL
Download data sheet
- Each mask is Individually packaged
- KN95/ FFP2 protection class III category
- Production standards in compliance with the regulation EN149:2001+A1:2009
- 4-ply mask with internal filtering layer in “non-woven” fabric
- Filtration efficiency ≥95
- Box of 60 pieces in single packs of 30 pieces each.
- Product marketed in derogation from INAIL
Download data sheet
- Each mask is Individually packaged
- KN95/ FFP2 protection class III category
- Production standards in compliance with the regulation EN149:2001+A1:2009
- 4-ply mask with internal filtering layer in “non-woven” fabric
- Filtration efficiency ≥95
- Box of 20 pieces in single packs of 5 pieces each.
- Product marketed in derogation from INAIL
Download data sheet
- Each mask is Individually packaged
- N95/ FFP3 con classe di protezione di Categoria III
- CE certificate issued UE 425/2016 e D.Lgs 17/2019
- Production standards in compliance with the regulation EN149:2001+A1:2009
- CE certificate issued by TÜV THÜRINGEN Shanghai Ltd
- Box of 20 pieces in single packs of 2 pieces each.
Download data sheet
- Extra strong, hypoallergenic and powder free nitrile glove
- Medical Device classification I, compliant with EC regulation – MDR 2017/745 EU
- CE Certificate of Conformity – EN ISO 374-1: 2016; EN374-4: 2013; EN374-5: 2016; EN420: 2013 + A1: 2009
- Category III PPE compliant with directive 93/42 / ECC – 2016/425
- CE Certificate of Conformity – PPE issued by : Kargilihanbaba Koyu2 OSB
- Durability: 5 years to production data
Download data sheet
- Extra strong, hypoallergenic and powder free nitrile glove
- Medical Device classification I, compliant with EC regulation – MDR 2017/745 EU
- CE Certificate of Conformity – EN ISO 374-1: 2016; EN374-4: 2013; EN374-5: 2016; EN420: 2013 + A1: 2009
- Category III PPE compliant with directive 93/42 / ECC – 2016/425
- CE Certificate of Conformity – PPE issued by : Kargilihanbaba Koyu2 OSB
- Durability: 3 years to production data
Download data sheet
- Extra strong, hypoallergenic and powder free nitrile glove
- Medical Device classification I, compliant with EC regulation – MDR 2017/745 EU
- CE Certificate of Conformity – EN ISO 374-1: 2016; EN374-4: 2013; EN374-5: 2016; EN420: 2013 + A1: 2009
- Category III PPE compliant with directive 93/42 / ECC – 2016/425
- CE Certificate of Conformity – PPE issued by : Emergo Europe
- FDA 510K + CHEMO approved
Download data sheet
- Compliant with Medical Devices Directive 93/42 / EEC Annex VII
- Box of 100 pieces, ambidextrous with rolled edge
- Sizes: XS – S – M – L – XL
- DOP, DEHP-free and latex-free is also an excellent alternative for those suffering from type I allergies
Download data sheet
- Compliant with Medical Devices Directive 93/42 / EEC Annex VII
- Classification: Class I CE
- Sizes: M and L – Color: blue – packs of 100 pieces, ambidextrous
- Brand name: Setino – Manufacturer: Hongray
- DOP, DEHP-free and latex-free. Ideal for type I allergy sufferers
Download data sheet
- Complies with EN 455 – EN 374 – ASTM D5250 – FDA510 (K)
- AQL 1.5 medical grade
- Box of 100 pieces, ambidextrous with rolled edge
- Sizes: XS – S – M – L – XL
- DOP, DEHP-free and latex-free. Ideal for type I allergy sufferers
Download data sheet
- Complies with EN 455 – EN 374 – ASTM D5250 – FDA510 (K)
- AQL 1.5 medical grade
- Box of 100 pieces, ambidextrous with rolled edge
- Brand name: Intco
- Sizes: XS – S – M – L – XL
- DOP, DEHP-free and latex-free. Ideal for type I allergy sufferers
Download data sheet
- Class I Medical Device (MDD) compliant with: 93/42 / ECC MDD Annex V
- Compliant with UNI EN 14126: 2004, UNI EN 13688: 2013, UNI EN 1149-5
- CE Certificate of Conformity issued by TÜV SÜD Product Service GmbH
- EC Representative (EC Representative) DISPOMED
- Composition: SMMS
- Box of 32 pieces
Download data sheet
- 45 Gram sterile surgical gown
- Class I Medical Device (MDD) compliant with: 93/42 / ECC MDD Annex V
- Compliant with UNI EN 14126: 2004, UNI EN 13688: 2013, UNI EN 1149-5
- CE Certificate of Conformity issued by TÜV SÜD Product Service GmbH
- EC Representative (EC Representative) DISPOMED
- Composition: SMMS
- Box of 32 pieces
Download data sheet
SURGICAL GOWN
Sterile Medical Device 45gr.
- 45 Gram sterile surgical gown
- Class I Medical Device (MDD) compliant with: 93/42 / ECC MDD Annex V
- Compliant with UNI EN 14126: 2004, UNI EN 13688: 2013, UNI EN 1149-5
- CE Certificate of Conformity issued by TÜV SÜD Product Service GmbH
- EC Representative (EC Representative) DISPOMED
- Composition: SMMS
- Box of 32 pieces
Download data sheet
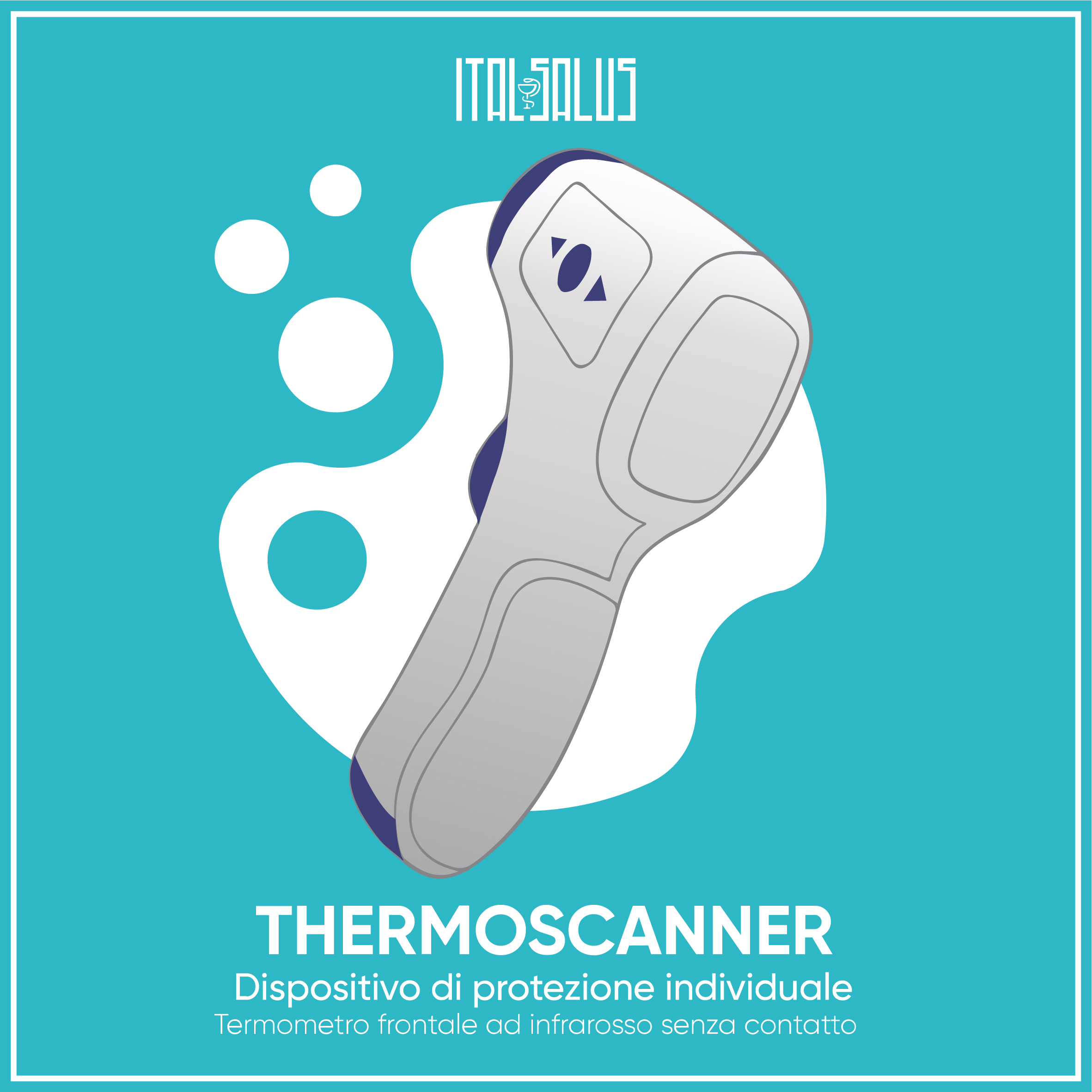
THERMOSCANNER
Non-contact infrared forehead thermometer
- CE and FDA certified
- Measurement distance: 1 – 15 cm
- Measurement time: less than 1 second
- Accuracy: ± 0.2 ° C in the measuring range 32 ° C ~ 43 * C
- Conditions: 10/40 ° C; relative humidity <85%; atmospheric pressure: 70kpa ~ 106kpa
- Power supply: DC3V, 2 * AAA batteries
Download data sheet